Background: Understanding the symptoms and quality of life (QOL) over time of adults with follicular lymphoma (FL) is important for treatment decision-making and clinical management. However, there are limited population-level data on the long-term QOL of adults with FL. We aim to describe the real-world, long-term QOL of adults with newly diagnosed FL up to 5/6 years after diagnosis.
Methods: We used the Mayo Clinic/Iowa Molecular Epidemiology Resource (MER) and the multi-institutional Lymphoma Epidemiology of Outcomes (LEO) to identify adults with grade 1-3A FL who completed QOL surveys at baseline and follow-up. Participants with lymphoma were prospectively enrolled within 9 months of diagnosis in the MER cohort from 2002-2015 and within 6 months of diagnosis in the multi-institutional LEO cohort study across 8 cancer centers from 2015-2020. The LEO/MER cohort studies systematically collected information on disease status, QOL, health behaviors, and functional assessment. Treatments, disease relapses, and deaths were verified by medical record review. Treating physicians determined clinical management. QOL was measured using the Functional Assessment of Cancer Therapy-General (FACT-G) at years 1, 2, 3, and 5/6 post-diagnosis. A higher FACT-G total score (range 0-108) indicated better QOL across 4 subscales (range): physical (0-28), social/family (0-28), emotional (0-24), and functional (0-28) well-being. We categorized participants based on their frontline management at time of diagnosis: “observation” (surveillance), “treatment” (systemic treatment with immunotherapy +/- chemotherapy), and “local” (radiation) groups. We employed a generalized linear mixed model to evaluate and compare the changes in QOL scores (as a continuous variable) from baseline for the observation, treatment, and local groups, adjusting for sex, race/ethnicity, age, FLIPI risk, and cohort (i.e., LEO or MER). We also evaluated QOL changes by FLIPI risk.
Results: Our study included 1,544 participants with FL and QOL data. At the time of enrollment, median age was 61 years (range 19-91), 88% were non-Hispanic White, and 49% were female. Based on initial management, 529 (34%) were in the observation group, 880 (57%) were in the treatment group, and 135 (9%) were in the local group. FLIPI risk was high for 17% in the observation group, 29.3% in the treatment group, and 3% in the local group.
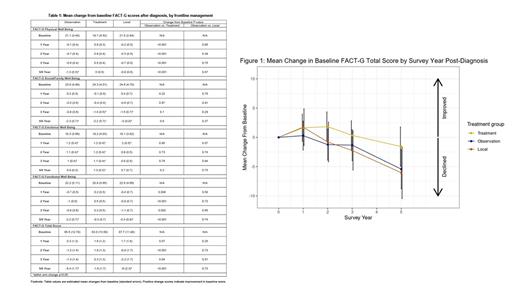
The mean (standard deviation) baseline FACT-G total score was lowest in the treatment group with the following baseline scores: 86 (13) for the observation group, 83 (14) for the treatment group, and 88 (12) for the local group (Table 1). The observation and local groups reported a greater worsening of their baseline FACT-G total score vs the treatment group at both the 2-year (-1.2, -0.8 vs +1.8, respectively) and 5/6 year (-5.4, -6 vs. -1.6, respectively) timepoints (Figure 1). This appeared to be driven by worsened physical and functional well-being in the observation (-1.3, -2.2, respectively) and local (-0.9, -2.4, respectively) groups vs the treatment group (0, -0.3, respectively). These differences were statistically significant between the observation vs treatment groups. There were no statistically significant differences in the other subscales, i.e., social/family and emotional well-being, by frontline management. Social/family well-being decreased across all groups by 5/6 years (-2.3 in observation, -2.2 in treatment, and -3 in local, p<0.05). Regardless of FLIPI risk, the total FACT-G scores decreased within each FLIPI risk group at 5/6 years (-4.4 for low, -3.8 for intermediate, and -4.9 for high, p<0.05). This was primarily due to worsened social/family well-being (-2.4 for low, -2.2 for intermediate, and -3 for high, p<0.05) at 5/6 years. There was no statistically significant difference in QOL between FLIPI risk groups.
Conclusion: This is one of the first and largest studies with real-world longitudinal QOL data for FL. Our study suggests that frontline systemic treatment initially improved QOL and resulted in a lower degree of QOL decline over time. Social/family well-being decreased over time regardless of frontline management or FLIPI risk. Further research is warranted to explore the impact of timing and specific systemic treatments on QOL, incorporate the patient/caregiver experience to address social/family well-being, and identify the clinical significance of our findings.
Disclosures
Tsang:Poseida Therapeutics: Current holder of stock options in a privately-held company. Bommier:LYSA/ELI: Research Funding; ITMO/AvieSan: Research Funding; INSERM: Current Employment; Philippe Foundation: Other: Mobility funding; Institut Servier: Research Funding. Casulo:Follicular Lymphoma Foundation: Other: Leadership role; Abbvie: Consultancy; Verastem: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; SecuraBio: Research Funding; Gilead Sciences: Research Funding; GenMab: Research Funding; Genentech: Consultancy, Research Funding; Lymphoma Research Foundation: Other: Leadership Role. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Pophali:SeaGen: Honoraria. Wang:Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Koehler:AbbVie: Consultancy, Other: Advisory Board; Jannsen: Other: Advisory Board; Astra Zeneca: Other: Advisory Board. Munoz:Portola: Research Funding; TG Therapeutics: Consultancy; Lilly/Loxo: Consultancy; Merck: Research Funding; MEI: Consultancy; Epizyme: Consultancy; Beigene: Consultancy, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy; Genmab: Consultancy; ADC Therapeutics: Consultancy; Millennium: Research Funding; OncView: Honoraria; Targeted Oncology: Honoraria; Kyowa: Honoraria, Speakers Bureau; Alexion: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Curio: Honoraria; Physicians' Education Resource: Honoraria; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Verastem: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Celgene: Research Funding; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding. Parikh:AbbVie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Accerta Pharmaceuticals: Research Funding; Bristol Myers Squibb-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim Pharmaceuticals Incc: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Sunesis: Research Funding; Vincerx: Research Funding. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Witzig:ADC: Membership on an entity's Board of Directors or advisory committees; Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Kura Oncology: Research Funding. Nowakowski:Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Selvita Inc: Consultancy; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Zai Lab Limited: Consultancy; ADC Therapeutics: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy; Kymera Therapeutics: Consultancy; MEI Pharma: Consultancy; Seagen: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis: Consultancy; Abbvie: Consultancy; TG Therapeutics: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ansell:Affirmed: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research. Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Habermann:BMS: Research Funding; Genentech: Research Funding; sorrento: Research Funding. Lossos:Adaptive: Honoraria; NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy; LRF: Membership on an entity's Board of Directors or advisory committees. Kahl:Abbvie: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria, Research Funding; ADCT: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Flowers:4D: Research Funding; V Foundation: Research Funding; Gilead: Consultancy, Research Funding; Adaptimmune: Research Funding; Nektar: Research Funding; Amgen: Research Funding; Beigene: Consultancy; Cellectis: Research Funding; Genentech Roche: Consultancy, Research Funding; Pharmacyclics: Research Funding; Genmab: Consultancy; Allogene: Research Funding; Acerta: Research Funding; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Pfizer: Research Funding; Karyopharm: Consultancy; Novartis: Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Morphosys: Research Funding; Iovance: Research Funding; Kite: Research Funding; Jannsen Pharmaceuticals: Research Funding; Spectrum: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Guardant: Research Funding; SeaGen: Consultancy; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Pharmacyclics Jansen: Consultancy; CPRIT Scholar in Cancer Research: Research Funding; Bayer: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Cerhan:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genmab: Research Funding; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal